
This study reports the application of hydrophobic coating on natural leather through plasma polymerization method and the hydrophobic behavior of the substrate . natural leather
Examines plasma coated. The combination of silicon hexane, methyl disiloxane (HMDSO), inert gas argon (Ar) and toluene were used to create surface hydrophobicity on the leather substrate. The results of water contact angle and adsorption time showed that hydrophobicity
The surface of the natural leather sample was clearly improved after the polymerization of HMDSO plasma on the surface of the material. XPS analysis showed that the plasma polymerization of HMDSO toluene compound caused a significant increase in the atomic percentage of Si compound, which is responsible for the property
It is superficial hydrophobicity. These atomic ratios showed that the highest amount of Si and the lowest amount of N, obtained with 100% HMDSO plasma process, confirm the higher degree of plasma angle polymerization than other plasma processes. The decrease observed in
The surface energy of natural leather samples after plasma deposition indicates an improvement in surface hydrophobicity.
Hydrophobic and superhydrophobic surface operations on a variety of substrates for applications such as easy-to-clean textile surfaces have attracted increasing attention in recent years. In general, hydrophobic and superhydrophobic surfaces are produced both by creating a rough structure on the hydrophobic surface, which has a contact angle of more than 90 degrees, and by modifying the rough surface with low-surface free energy materials.
Easy care properties are created on the fabrics of interior appliances (curtains and sofas) and clothing that should prevent dust and stains, by the use of water and oil repellents such as paraffin, polysiloxane and fluorocarbon polymers. Conventional methods, including the use of solvents and organic reagents, mainly wax or wax emulsions, alkene derivatives and hydrophobic resin additives to achieve water repellency, can cause environmental problems due to the disposal of harmful effluents in operating baths. .
In the plasma process, small amounts of reactants are used and due to the short operating times, few materials need to be disposed of, which has environmental benefits. Plasma polymerization of hexamethyl disiloxane (HMDSO) on polyester and cotton blends was studied to achieve superhydrophobic properties.
The formation of a water repellent film on polyester fiber was reported by plasma polymerization using a mixture of argon gas and HMDSO gas phase at atmospheric pressure. Levasseur and colleagues reported the improvement of hydrophobicity on wood surfaces of sugar maple and black spruce using He / HMDSO plasma at atmospheric pressure, controlled by the dielectric barrier. A slight increase in water contact angle was also obtained on natural leather after plasma treatment, which was performed in tetrafluoromethane (CF4) atmosphere.
In the plasma polymerization process, monomers are converted to plasma polymers by the formation of gas phase free radicals, and their recombination at radical sites during film growth results in amorphous, crosslinked, and therefore insoluble, stable structures. It becomes heat, and mechanically hard.
The films produced in this way offer the possibility of creating stainless steel surfaces, scratch-resistant coatings, chemical barrier coatings, and water-repellent coatings.
Hexamethyl disiloxane (3 (HMDSO, (CH3)) 3-Si-O-Si- (CH3), due to the retention of CH3 groups within the Si-O network, is mainly used to achieve hydrophobic surfaces by plasma polymerization.
Wang reported that the improvement of water repellency on cotton fiber surfaces through plasma polymerization, due to the chemical functional groups Si- (CH3) 2, Si-O-Si and Si-C produced from HMDSO fragmentation and reactions Plasma chemistry was between the substrate and the plasma. It was reported that the resulting Si-O-C from the Si-O-Si functional groups broken down by plasma electron collision is then attached to cellulose by chemical bonds of Si-O-Cellulose. In our study, Si-O functional groups, which were also observed after plasma surgery, indicated that the film containing the most primary Si-O functional groups successfully sat on the surface of natural leather.
Although there have been a number of attempts to increase the hydrophobicity of textile-based substrates through plasma treatment, little study has been done on plasma deposition on natural leather. In the present study, the non-corrosive and non-toxic silicone compound hexamethyl disiloxane (HMDSO) was selected as the plasma coating material.
The inert gas argon (Ar) was used as the carrier or carrier for the monomer, and toluene, an aromatic hydrocarbon, was used to reduce the surface polarity due to its non-polar properties. The purpose of this study is to use the plasma process as an ecological complement method to create surface hydrophobicity and easy care properties on natural leather, which has applications in clothing, home appliances and in cars.
All experiments were performed at relative humidity of 65% and 21 ° C.
Materials
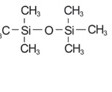
In this study, hexamethyl disiloxane (HMDSO) obtained from Aldrich Company (98% purity) was used without any purification. The chemical structure of HMDSO, which was coated on a natural leather substrate by the plasma polymerization method, is shown in Figure 1.
Toluene was obtained from Al-drich (purity 8.99%). Argon was used as the carrier gas at a flow rate of 500 cm3 / min and HMDSO was maintained at 25 ° C. The material was bubbled by argon and fed to the plasma tank. The natural leather specimens were wet white goat skins, tanned with aluminum and zirconium-based tanning materials for light interior applications, weighing 400 grams per square meter, and 1 mm thick. The samples were supplied by a natural leather manufacturer.
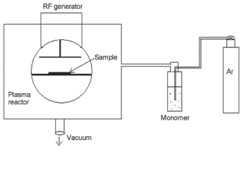
Figure 1- Chemical structure of hexamethyl nedisiloxane
Plasma operation
The outline of the low pressure plasma system used for natural leather surface treatment is shown in Figure 2. The system is a 13.56 MHz radio frequency (RF) source within an L-C matching unit with a maximum power of 100 watts. All samples in this study were processed in plasma power of 20-20 watts. A mixture of HMDSO toluene, bubbled by argon gas, was injected into the reactor tank. The operation was performed at a pressure of 40 Pascals for 10–20 seconds.
The experiments were performed by changing the ratio of HMDSO toluene mixture. Combinations of 100% HMDSO and 1: 3,1: 1 toluene HMDSO were used during plasma polymerization on natural leather samples.
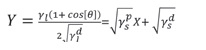
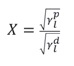
In order to determine the conditions that provide the maximum contact angle of water and therefore the best hydrophobic results, plasma polymerization was performed at different capacities and times of plasma operation. Plasma-free specimens have been cited as practical examples.
Figure 2 – Outline of the plasma system used in low pressure,
Water contact angles and adsorption times on natural leather samples were measured before and after polymerization of HMDSO plasma and HMDSO toluene mixtures using contact angle meters from KSV Instruments. Finland equipped with CAM 200 software.
Water contact angles were measured at different time intervals and changes in water contact angles were observed and compared with the measured angles on the untreated sample. Distilled water droplets with a constant volume of 20 μl were injected on the surface of the sample using a syringe. The system was equipped with a CCD camera and computer-based data processing and acquisition software. Drop photos were taken by the camera at a speed of 1 frame per second.
Stagnant water contact angles were automatically measured by the software using Young-Laplace curve matching based on photographed drop profiles. 5 different contact angle measurements were taken from each sample and the measurements were repeated 5 times for one sample and then the mean values were calculated.
Plasma power was changed from 20 to 100 watts; Plasma operating times of 30, 60, 90 and 120 seconds were used during the experiments. Repeatability of contact angle experiments was performed.

![]()
Changes in surface hydrophobicity of various substrates through plasma deposition were also investigated by measuring surface free energy before and after plasma operation. Surface free energy measurements were performed on the contact angle of the KSV Instruments. The contact angles of distilled water, diidomethane or methylene iodide, ethyl neglycol, and form amide were measured on natural leather substrates. The total surface free energy (Y) for the natural leather sample was calculated by CAM 200 software based on the Oven and Wendt equation, which relates the contact angle (θ) to the solid surface energy (γs) and liquid (γl) and to the surface tension Makes. The principal relation of a linear equation is Y = mX + b, in which m is the slope and the point of intersection b, given by the square root of the free energy component of the solid surface.
Easy or quick cleaning properties of treated and untreated natural leather stains were tested. Rapid staining test was performed using a standard Taber Industries device. The stained test specimen was fixed to the base of the device and a standard plain white rectangular piece was moistened with distilled water and mounted on the finger of the device with a diameter of 16 mm. The tip of the device was rubbed on the sample at a pressure of 250 g, following a straight path approximately 100 mm long with each arm movement. A total of 20 movements were used during the test to compare stain removal from treated and unprocessed surfaces. The experiments were repeated twice for each type of stain, namely auto ink and mustard. Remaining stain images were taken on treated and unprocessed samples for visual comparison.
![]()
![]()
The NovaTM NanoSEM system of FEI was used to analyze the surface morphology of unprocessed and polymerized natural leather samples from plasma.
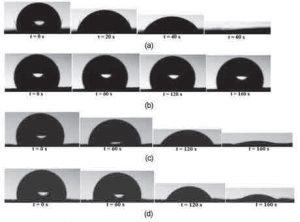
Figure 3 – Absorption of water droplets against time t, not on sample a and samples polymerized with 1: 1 toluene plasma at 80 watts plasma power and 90 seconds plasma operation time.
XPS analysis was performed to identify the chemical composition of the surface of natural leather samples polymerized with plasma. XPS measurements were performed using a high-performance XPS spectrometer from Thermo Scientific. The pressure in the analysis tank was kept at 7-10 Pascals or less during the analysis and the size of the analyzed area was 7.7 mm.
Different combinations of 100% 3: 1, HMDSO and HMDSO 1: 1 toluene was used during plasma polymerization experiments and the surface hydrophobicity obtained from different plasma compounds was compared.
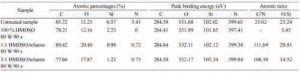
Figure 3 shows the wetting behavior of water droplets deposited on unprocessed and polymerized natural leather samples at 80 watts plasma power and 90 s plasma operating time for different combinations of HMD-SO toluene mixture. As can be seen from Figure a3, on the surface of the untreated specimens, the water contact angle was reduced from 105 to zero degrees for 60 seconds.
100% non-polar HMDSO was used for plasma deposition on natural leather substrate and water contact angles were measured to analyze changes in surface hydrophobicity. As can be seen from Figure b3, the contact angle of water decreased from 107 to 105 degrees in 160 seconds, indicating a very small change in the contact angle.
The water droplet remained on the surface and the contact angle of the water after 700 seconds was 90.3 degrees. The contact angle of water began to decrease to less than 90 degrees over time, and at 2800 seconds, the contact angle reached zero degrees, as well as the evaporation of the drop and the contraction of the drop occurred. Compared to the untreated sample, surface hydrophobicity was obtained due to the hydrophobic coating.
Toluene, a non-polar aromatic hydrocarbon, was mixed with HMDSO in a 1: 3 mixing ratio and placed on a natural leather substrate. As can be seen from Figure c3, after the HMDSO-toluene mixture settled in a 1: 3 ratio on the sample surface through plasma polymerization, the contact angle was reduced from 100 to zero degrees in 160 seconds. The water uptake time of 60 s on the untreated sample was increased to 160 s on the treated sample with a 1: 3 HMDSO / toluene mixing ratio. However, due to the lower adsorption time compared to the results obtained for 100% HMDSO, surface hydrophobicity could not be achieved with a 1: 3 toluene HMDSO mixing ratio. Then 1: 1 HMDSO toluene mixing ratio was used for plasma deposition on natural leather substrate. Figure c3 shows the wetting behavior of water droplets placed on a natural leather sample after 1: 1 toluene HMDSO plasma polymerization. The contact angle decreased from 90 to zero degrees in 160 seconds after the drop landed on the surface.
The uptake time of water after plasma placement of the 1: 1 toluene HMDSO mixture was similar to the water uptake time for the 1: 3 toluene HMDSO mixing ratio. The results of water contact angle and adsorption time showed that the surface hydrophobicity of natural leather samples was clearly improved after polymerization of HMDSO plasma on the surface of the material.
This result could be related to the hydrophobic surface formed by silicon compounds with HMDSO plasma treatment.
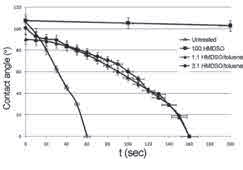
As can be seen from Figure 4, samples with polymerized plasma showed higher contact angle values over time compared to unprocessed samples. The contact angle values obtained through 1: 3 plasma polymerization and 1: 1 toluene HMDSO were comparable, although for the HMDSO toluene mixing ratio of 1: 3, the initial contact angle was higher at zero. Samples with 100% HMDSO polymerized plasma showed the highest contact angle, approximately 107 ° at zero time, which remained above 90 ° even after 700 seconds.
Figure 4: Time versus contact angle for 100% HMDSO, HMDSO toluene compounds
1: 1 and HMDSO toluene 1: 3 for 80 watt plasma power and 90 seconds operation time.
The contact angle results clearly indicated that the operation improved the hydrophobicity of natural leather. 100% HMDSO plasma polymerization resulted in a hydrophobic film coating for 80 watts of applied plasma power and 90 seconds of operation time. Due to the hydrophobic layer deposited on the natural leather substrate, water droplets over time tend to remain on the treated specimens instead of penetrating the structure. The motion of the water droplets was controlled by the surface properties, which are the irregular surface energy and surface morphology of natural leather. In order to observe the adsorption behavior of water droplets, all 5 samples of unprocessed and plasma coated natural leather were weighed using 100% HMDSO percent, carefully before removing 1 ml of distilled water on each sample.
Water droplets remained on the samples for 15 minutes. Then, the liquid was removed using absorbent paper. The samples were then weighed again. The calculated added weights due to the water absorption of the substrates are presented in Table 1. The weight gain on the plasma-coated natural leather substrate was only about 1% due to the absorption of the droplet, which indicated that the water droplet tended to remain on the surface instead of penetrating into the structure.
Table 2 shows the results of water contact angle and adsorption time on natural leather for untreated sample and plasma polymerized sample using 100% HMDSO with a processing time of 90 seconds, when the plasma power changed from 20 to 100 watts. Gives. The results showed that the increase in plasma power will lead to an increase in surface hydrophobicity based on the time absorption results.
However, when the plasma power increased from 80 to 100 watts, the absorption time decreased to 230 seconds. Contact angles at t = 0 were comparable at different plasma power values. Overall, the highest improved surface hydrophobicity was obtained at 80 watts plasma power; which led to the successful setting of the silicon compound, responsible for creating water repellency, on the natural leather substrate.
Table 3 shows the results of water contact angle and adsorption time on untreated and plasma-treated natural leather at various plasma operating times from 30 to 120 seconds. The sample was coated with 100% HMDSO by plasma plating at 80 watts. The maximum water uptake time was achieved during plasma operation of 90 seconds. The increase in plasma operation time resulted in a slight decrease in water contact angles at t = 0 s. The water uptake time increased with the plasma operation time to about 90 seconds. When the plasma operating time increased from 90 seconds to 120 seconds, the absorption time dropped to 1100 seconds. Overall, the results showed that the most improved surface hydrophobicity was obtained at 80 watts plasma power and 90 seconds operation time. The results of free energy of unprocessed and plasma-treated natural leather surface with 80-watt plasma power and 90-second operation time are shown in Table 4.
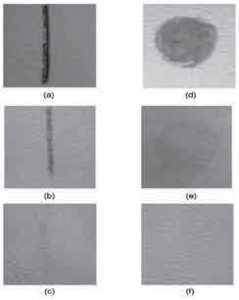
Figure 5 shows the stain removal results for unplaced and plasma-treated natural leather samples. Stains were created on the surface using auto ink and mustard, which were allowed to dry for about 12 hours before being removed. After applying 20 motions to the thermometer, the photographs clearly showed that the stains still remained on the untreated specimens, while the specimens were barely visible on the plasma polymerized specimens.
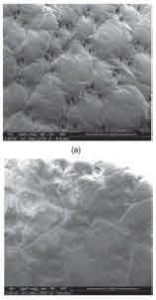
Figure 6 shows scanning electron microscopy images of a natural leather sample before and after plating with HMDSO via plasma. On plasma-coated material, the gaps and holes created by the hair glands were covered by the plasma-coated material, and a thin film was observed on the remaining areas of the background.
Table 3- Water contact angle at t = 0 seconds and adsorption time on untreated and plasma coated samples with 100% HMDSO for 80 watt plasma power
Table 4 – Free energy results of the surface of untreated and plasma-treated samples
The surface chemistry of untreated and plasma coated natural leather samples at 80 watts and 90 seconds plasma operating time was studied by XPS and the results are summarized in Table 5. The main components were unprocessed surface and plasma coated surface, carbon, oxygen, silicon and a small percentage of nitrogen. The peaks of C 1s, O 1s, Si 2p, and N 1s for unprocessed and plasma-coated samples were approximately 285, 532, 102, and 400 electrololes, respectively. For plasma polymerized samples, the locations of Si, O, C, and N peaks did not change significantly compared to the treated sample.
The atomic percentages of silicon, oxygen and carbon components for the surfaces of untreated natural plasma and polymerized with plasma are shown in Table 5. There was 0.57% silicon on the surface of the untreated sample, which may have come from the fabrication process or may be due to the presence of silicon in the XPS assay tank.
Figure 5. Stains of (a) auto ink and (d) mustard stains on untreated samples.
After 20 movements of the odometer; Auto ink stain left on Samples (b) untreated and (c) treated with plasma, residual mustard stain
Unoperated (e) and (f) plasma-treated specimens.
Figure 6. SEM photos of sample (a) unprocessed, (b) coated
Plasma using 100% HMDSO in time
90 second plasma operation and 80 watt plasma power (× 100)
Compared to untreated samples, the atomic percentage of silicon on samples polymerized with HMD-SO plasma, 100% HMDSO / toluene 1: 3 and 1: 1, from 0.57% to 2.23%, 0.98%, respectively and increased by 1.23%. The highest percentage of silicon was transferred to the substrate surface through 100% HMDSO. Atomic oxygen content on 100 HMDSO samples the percentage of plasma polymerized decreased from 13.25% to 12.16% compared to the untreated sample.
Table 5 – Elemental components of plasma coated natural leather samples for different HMDSO toluene compounds
The decrease in atomic oxygen content could be the reason for the improved hydrophobicity obtained on the 100% HMDSO polymerized plasma sample. On samples with plasma polymerized with HMDSO toluene with a mixture ratio of 1: 1 and 1: 3 compared to unprocessed samples, the atomic percentage of oxygen from 13.25 to 20%, respectively Increased by 40 and 17.87%.
No nitrogen was observed on the surface of the sample treated with 100% HMDSO, indicating the deposition of a film of uniform thickness on the surface.
The percentage of nitrogen on the surfaces of the samples after plasma coating with HMDSO toluene 1: 1 and 1: 3, could be a sign that the monomer deposition on the leather surface was not uniform or the thickness of the applied layer was relatively thin. Compared to untreated samples, the atomic percentage of nitrogen on plasma coated samples with HMDSO toluene 1: 1 and 1: 3 decreased from 3.41% to 0.72% and 0.73%, respectively.
The atomic percentage of carbon also decreased with plasma polymerization. As can be seen from Table 5, the C / N atomic ratio for HMDSO toluene compounds with a mixing ratio of 1: 1 and 1: 3, showed that plasma polymerization produces a nitrogen-containing surface that is about 100 HMDSO not found. The O / Si atomic ratio showed that plasma polymerization produced a richer surface than Si due to the increase in silicon compounds with HMDSO plasma treatment.
These atomic ratios showed that the highest SI value and the lowest N value were obtained with 100% HMDSO plasma process, which confirms the higher degree of plasma polymerization than other plasma processes. In addition, the separation of the C1s peak curves for the untreated sample was matched by 3 peaks: one large peak of about 284.54 electrollects due to C = C bonds, the other peak of about 286.232 electroluttes belonging to CN bonds or CO and a small peak at approximately 58.288 eV due to C = O, CN, or C-O bonds.
On the plasma coated sample with 100% HMDSO, the C1s peaks were also matched with 3 peaks: one large peak at 284.41 eV due to C = C bonds, the other peak at about 286.06’s belonging to CO or C = O bonds. And a small peak at approximately 288.65 electron volts due to C = O or CO-H bonds. As can be seen from Table 5, the atomic ratio of nitrogen derived from natural leather on the sample surface decreased to zero% after 100% HMDSO plasma coating.
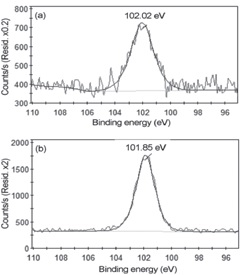
Separation of Si 2p peak curves can also be done to gain more insight into the chemical bonds present in the samples. Figure 7 shows Si 2p peaks for samples of unworked, plasma-coated natural leather. The unopened Si 2p peak has only one peak of about 102.02 electron volts, which corresponds to Si3N4 units. The Si 2p peak of the plasma-coated natural leather sample also has only one peak of about 85,101 electron volts, which corresponds to SiO or SiO2 units. This result could be due to the formation of a layer of new silicon compounds on the sample surface through plasma coating.
Figure 7. Si 2p peaks for (a) untreated sample and (b) treated sample with plasma using 100% HMDSO.
We proposed surface modification of natural leather substrates through plasma polymerization of different HMDSO toluene blends. Surface hydrophobicity was improved by the introduction of silicon atoms on the surface of natural leather. The formation of a layer of new silicon compounds on the surface through plasma coating increased the contact angle and water absorption time. Significant improvement in the rapid scaling of the stain was observed in the 100% HMDSO plasma polymerized sample compared to the untreated sample. The reduction in free energy levels of the total surface of plasma polymerized leather samples compared to the untreated sample indicated an improvement in surface hydrophobicity.
Placement of HMDSO with plasma at low pressure showed promising results in improving the easy cleaning properties of natural leather. Plasma deposition or coating can be used as a method of environmental (ecological) surface treatment among conventional methods in which solvents and organic reagents are used for surface hydrophobicity.
Collector: Mr. Masoud Hashemi – Today’s textile Magazine